Level l INTRACEPT Study
Demonstrates Highly Significant Results1
Purpose
To compare the effectiveness of the Intracept Procedure to standard care for the treatment of chronic vertebrogenic low back pain.
Study Design
- Prospective, randomized, multi-center
- 20 US sites
- 104 Patients; Randomized to Intracept (51) or standard care (53)
- Patients were evaluated at baseline and at 3, 6, 9, and 12 months post-treatment
- Skeletally mature patients with low back pain ≥ 6 months who had not responded to at least 6 months of conservative care
- All patients had Type 1 or Type 2 Modic changes at up to 4 vertebral bodies (L3-S1)
- Primary Endpoint: Between-arm comparison of the mean change in ODI from baseline to 3 months post-treatment
Key Findings
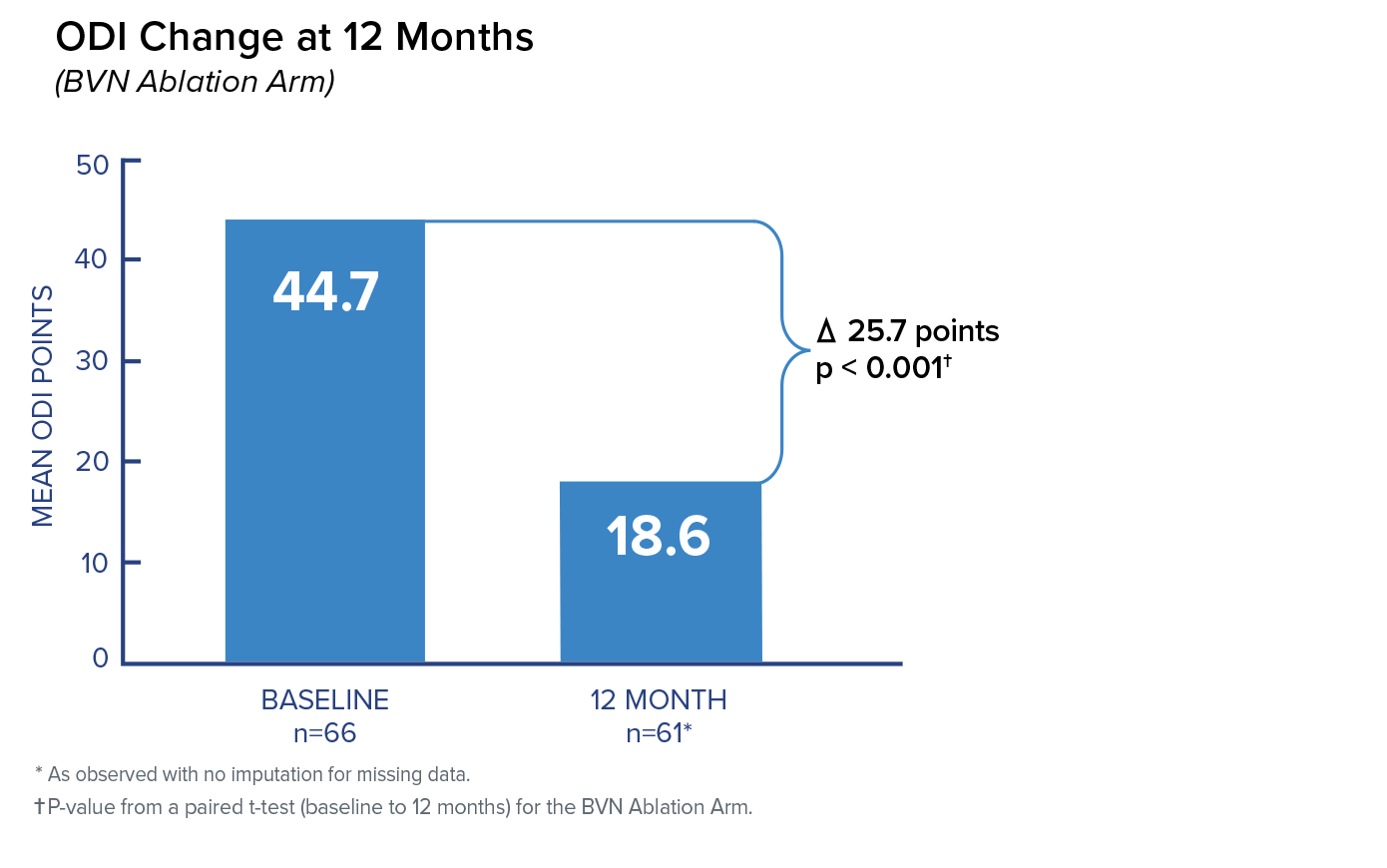
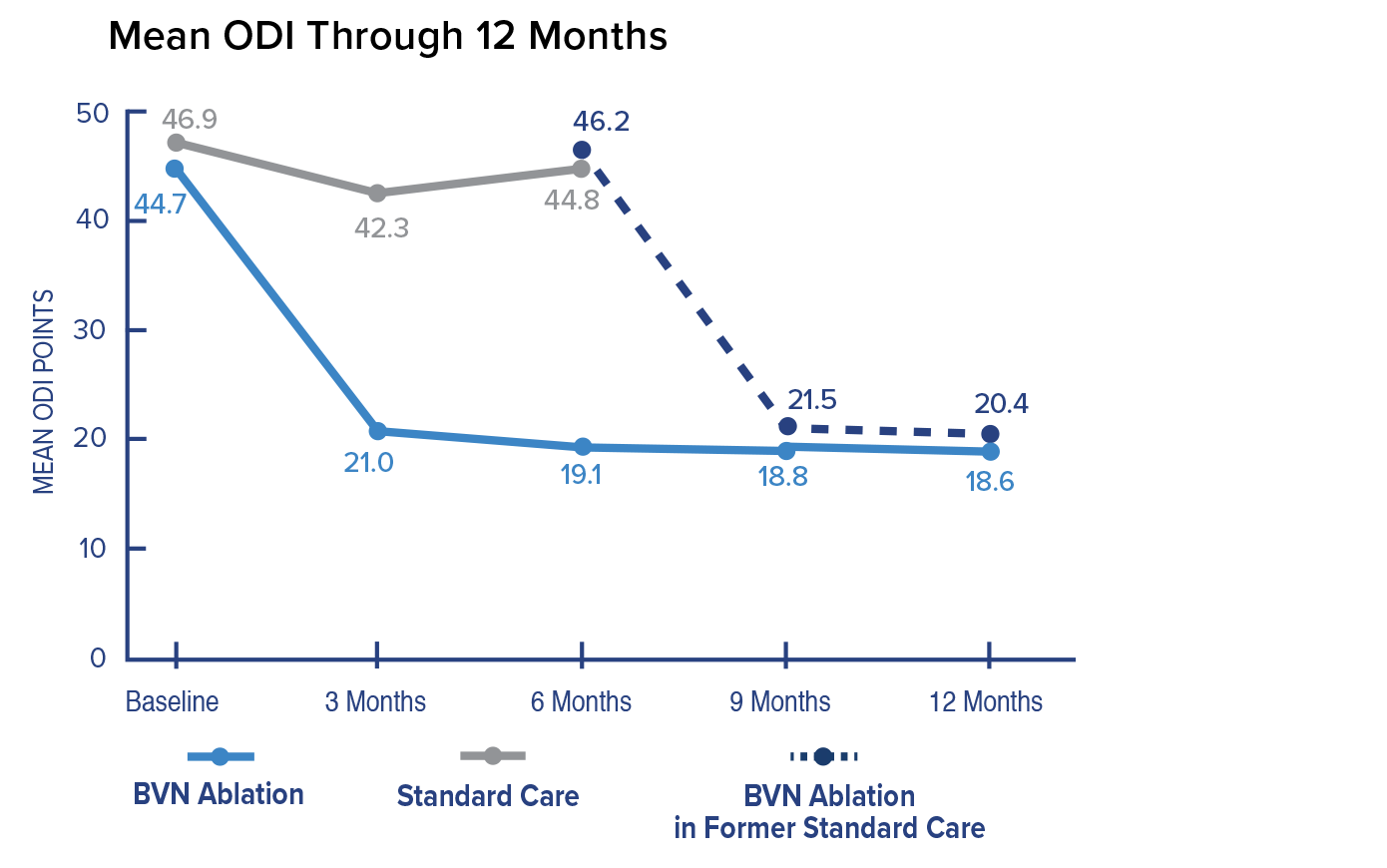
Primary Endpoint; Change in ODI at 3 Months
In the Intracept arm, patients reported a 25.3 mean improvement in ODI at 3 months compared to patients reporting a 4.4 mean improvement in the standard care arm. The difference between arms of 20.9 was highly significant.
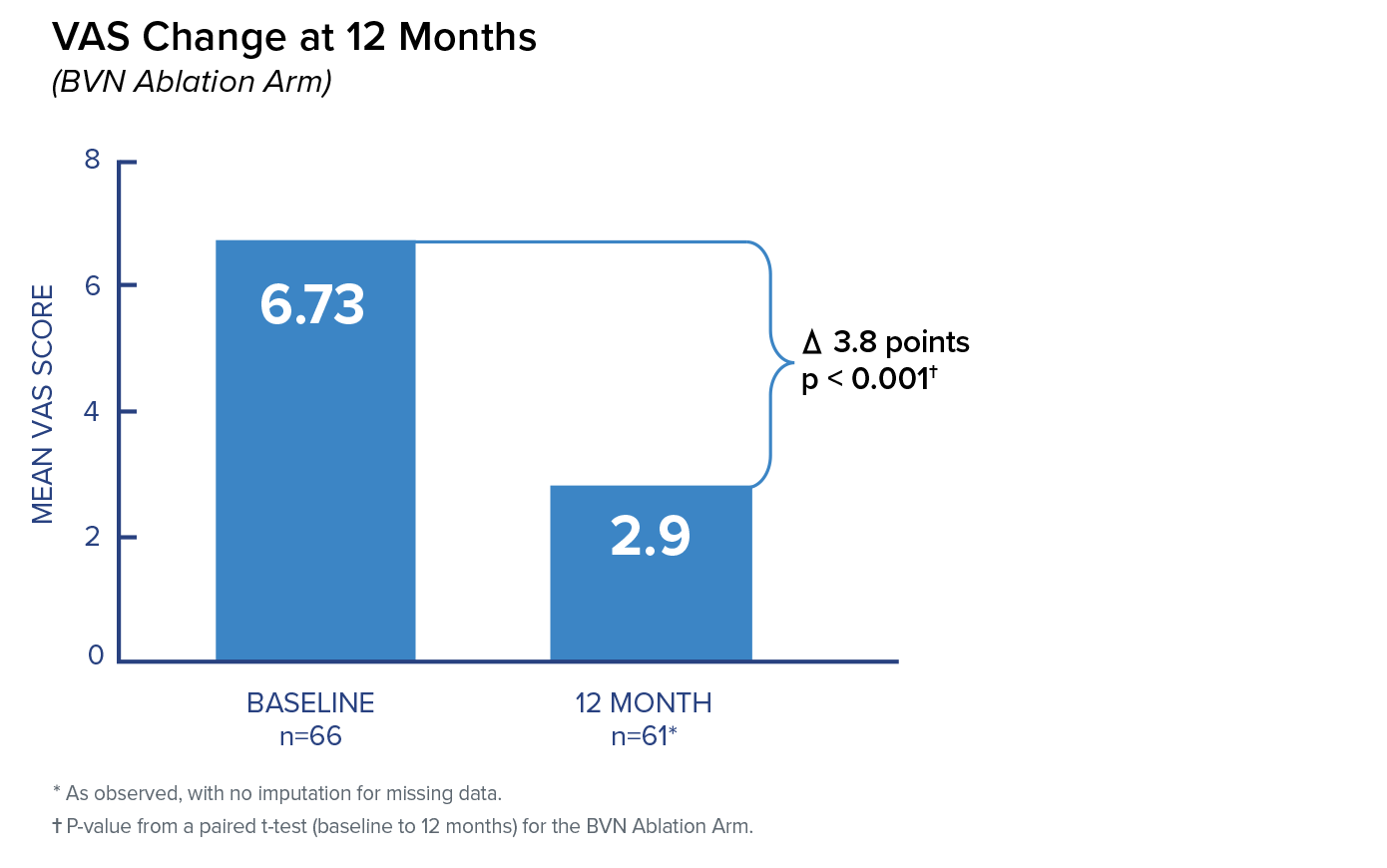
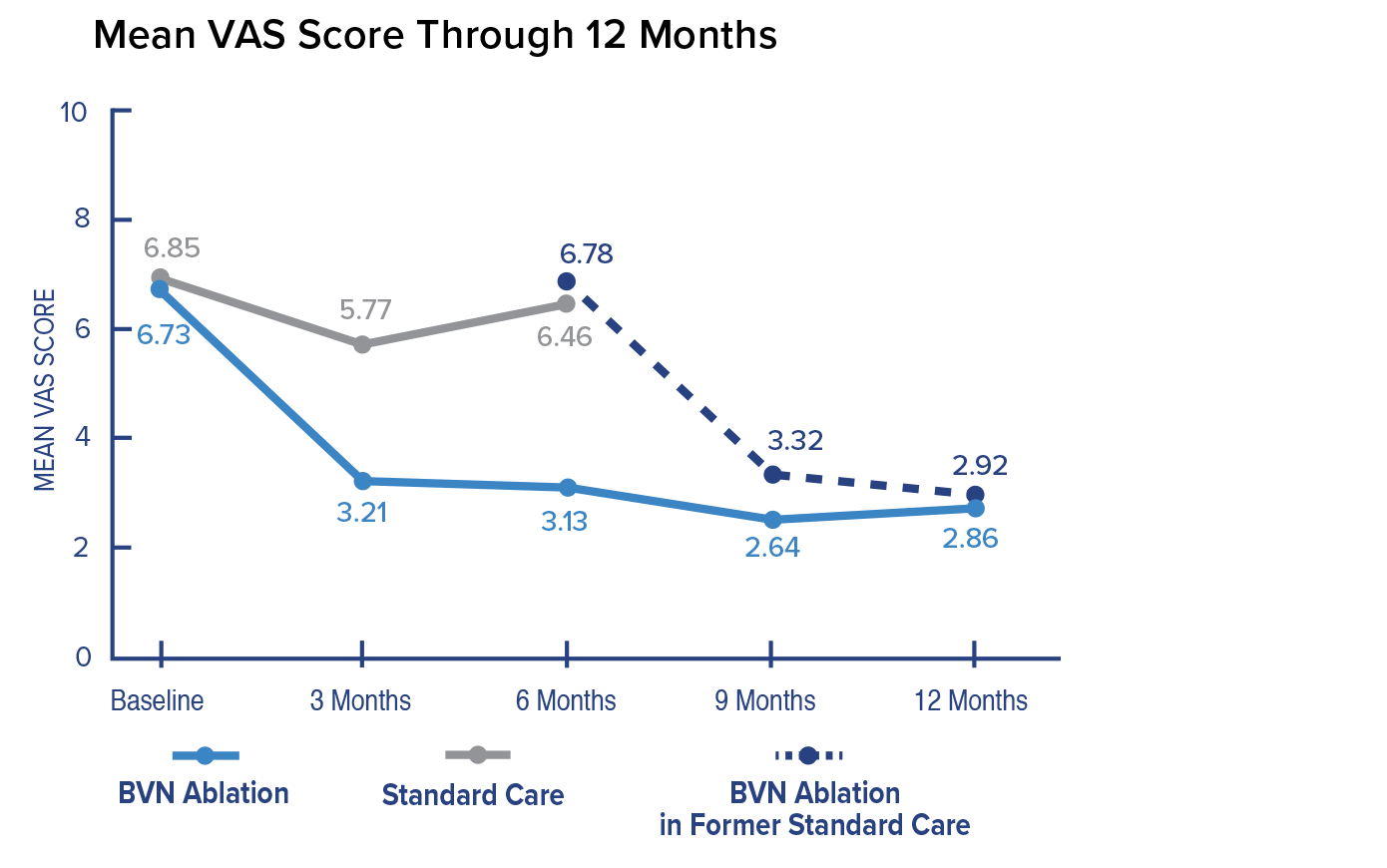
Change in VAS at 3 Months
In the Intracept arm, patients reported a 3.46 mean improvement in VAS at 3 months compared to patients reporting a 1.02 mean improvement in the standard care arm. The difference between arms of 2.44 was highly significant.
ODI Responder Rates – Baseline to 3 months
In the Intracept arm, responder rates at 3 months were highly significant compared to standard care at both ≥ 10-point and ≥ 20-point reduction in ODI.
VAS Responder Rates – Baseline to 3 months
In the Intracept arm, responder rates at 3 months were highly significant compared to standard care at both ≥ 1.5 cm and ≥ 2.0 cm decrease in VAS.
Safety Results
There were no device related adverse events. All procedure related adverse events were considered mild and resolved with oral medications.
Conclusion
The Intracept Procedure led to significant improvement of pain and function in patients with chronic vertebrogenic low back pain.