Hero Curved Left No Overlay
Relievant Announces Positive 12-Month Intracept Effectiveness Study Results From Community Based Practices
Relievant Medsystems, a privately-held medical device company transforming the treatment of Chronic Low Back Pain (CLBP), today announced the North American Spine Society Journal publication of results from an effectiveness study showing that the significant improvements in function and pain initially reported at an interim analysis of three months following the Intracept Procedure remained significant through one year.
Relievant Announces Publication of 5-Year Data Demonstrating Long Term Clinical Benefits of the Intracept Procedure in Chronic Low Back Pain Patients
Relievant Medsystems, a privately-held medical device company transforming the treatment of Chronic Low Back Pain (CLBP), today announced European Spine Journal publication of long-term data from the Level I SMART trial showing durability of improvements in pain and function beyond 5 years for patients treated with the Intracept procedure…
Relievant Announces Publication of Positive Results from Multi-Center Study of the Intracept® Procedure in Real-world Setting for Patients with Chronic Low Back Pain
Relievant Medsystems, a privately-held medical device company transforming the treatment of Chronic Low Back Pain (CLBP), today announced the publication of significant and clinically-meaningful outcomes from a prospective, open-label, single-arm, multi-center study in the European Spine Journal…
Relievant Announces Publication of Level l INTRACEPT Study
Relievant Medsystems, a privately-held medical device company focused on transforming the treatment of Chronic Low Back Pain (CLBP), today announced the publication of the Level I INTRACEPT Study results in The Spine Journal. The INTRACEPT Study is a Level I,...
Relievant Announces Publication of SMART Trial 24-Month Results
Relievant Medsystems, a privately-held medical device company developing minimally-invasive solutions for chronic low back pain (CLBP), today announced the publication of 24-month results from the SMART trial in the International Journal of Spine Surgery. A total of...
American Society of Spine Radiology Announces the Presentation of Results From the Level l INTRACEPT Study
Results from the pre-specified interim analysis of the landmark INTRACEPT randomized controlled trial were presented for first time on February 20, 2019 at the American Society of Spine Radiology (ASSR) meeting in Miami, Fla. The study demonstrated statistically and...
Relievant Announces Level l INTRACEPT Study Stopped Early for Superiority
Relievant Medsystems, a privately-held medical device company, developing minimally-invasive solutions for chronic low back pain (CLBP), today announced that a pre-specified interim analysis of the Level I INTRACEPT Randomized Controlled Trial (RCT) found a highly...
Relievant Announces Reimbursement Codes and APC Assignment for The Intracept® Procedure
Relievant Medsystems, a privately-held medical device company developing minimally-invasive solutions for chronic low back pain (CLBP), today announced that the Centers for Medicare & Medicaid Services (CMS) has established Healthcare Common Procedure Coding...
Relievant Announces First Commercial Intracept Procedures
Relievant Medsystems, a privately-held medical device company developing minimally-invasive solutions for chronic low back pain (CLBP), announced today the successful completion of the first commercial Intracept Procedures. The procedures were performed by Dr. Bradly...
Relievant Announces Completion of $58M Series E Financing to Support US Commercialization
Relievant Medsystems, a privately-held medical device company developing minimally-invasive solutions for chronic low back pain (CLBP), announced today the completion of a $58 million equity financing. The round was led by Endeavour Vision and included participation...
Hero Curved Left Overlay
Hero Curved Center
That’s Living
Proof.
Hero Curved Right
Welcome to the proof
of Intracept
Hero Image Text
Mattis Parturient Vestibulum Risus Sit
Morbi leo risus, porta ac consectetur ac, vestibulum at eros.
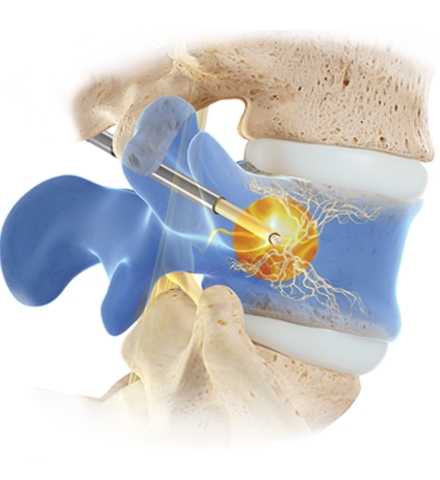
Image Text

Duis mollis, est non commodo luctus, nisi erat porttitor ligula, eget lacinia odio sem nec elit. Curabitur blandit tempus porttitor. Fusce dapibus, tellus ac cursus commodo, tortor mauris.
Morbi leo risus, porta ac consectetur ac, vestibulum at eros.
Chart Text
Mattis Parturient Vestibulum Risus Sit
Key Findings:
Vulputate Pellentesque Lorem Adipiscing
Key Findings:
Vulputate Pellentesque Lorem Adipiscing
Video Text
Mattis Parturient Vestibulum Risus Sit
Duis mollis, est non commodo luctus, nisi erat porttitor ligula, eget lacinia odio sem nec elit. Curabitur blandit tempus porttitor. Fusce dapibus, tellus ac cursus commodo, tortor mauris.
3 Column Image
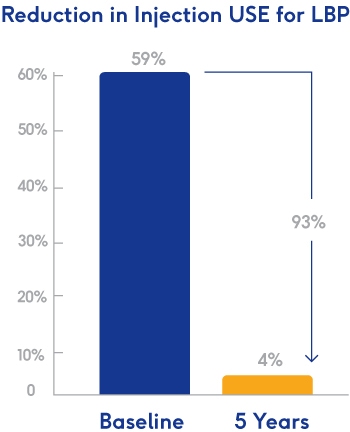
The Data Is Living Proof Too

Fewer Opiods
Maecenas sed diam eget risus varius blandit sit amet non magna. Cum sociis natoque penatibus et magnis dis parturient montes, nascetur ridiculus mus.

Fewer Opiods
Maecenas sed diam eget risus varius blandit sit amet non magna. Cum sociis natoque penatibus et magnis dis parturient montes, nascetur ridiculus mus.

Fewer Opiods
Maecenas sed diam eget risus varius blandit sit amet non magna. Cum sociis natoque penatibus et magnis dis parturient montes, nascetur ridiculus mus.
Curve Right Text
Mattis Parturient Vestibulum Risus Sit
Curve Left Text
Mattis Parturient Vestibulum Risus Sit
Curve Left Text Overlay
Icon Lists
Accordion Cards
How we’re living the proof.
Now in Effect: New Category | CPT Codes
Intracept Long Term Outcomes
New Patient Story
Your content goes here. Edit or remove this text inline or in the module Content settings. You can also style every aspect of this content in the module Design settings and even apply custom CSS to this text in the module Advanced settings.
Indications (Global)
As with any surgical procedure, there are risks and considerations associated with the Intracept Procedure. See important safety information below.
Physicians: See Indications, Contraindications, and Risks
The Intracept Procedure Intraosseous Nerve Ablation System is intended to be used in conjunction with radiofrequency (RF) generators for the ablation of basivertebral nerves of the L3 through S1 vertebrae for the relief of chronic low back pain of at least six months duration that has not responded to at least six months of conservative care, and is also accompanied by features consistent with Type 1 or Type 2 Modic changes on an MRI such as inflammation, edema, vertebral endplate changes, disruption and fissuring of the endplate, vascularized fibrous tissues within the adjacent marrow, hypointensive signals (Type 1 Modic change), and changes to the vertebral body marrow including replacement of normal bone marrow by fat, and hyperintensive signals (Type 2 Modic change).
Use of the Intracept Procedure Intraosseous Nerve Ablation System is contraindicated in:
- Patients with severe cardiac or pulmonary compromise
- Patients where the targeted ablation zone is < 10 mm away from a sensitive structure not intended to be ablated, including the vertebral foramen (spinal canal)
- Patients with active systemic infection or local infection in the area to be treated
- Patients who are pregnant
- Skeletally immature patients (generally < 18 years of age)
- Patients with active implantable pulse generators (e.g., pacemakers, defibrillators)
- Situations where unintended tissue damage may result, based on the clinical assessment by the physician
- Application with electrosurgical instruments NOT tested and specified for use with the Relievant RFG
Patients: See the Indications & Risks Involved
- The Intracept Access Instruments and the Intracept RF Probe are single patient use only. The Introducer Cannula, Diamond Stylet, Bevel Stylet, J-Stylet, Straight Stylet, Drill, and Probe may be used to treat up to a maximum of four (4) vertebrae (L3, L4, L5, S1) on a single patient, while the Curved Cannula may only be used on one (1) vertebra on a single patient.
- As with any surgical instrument, careful attention must be exercised to ensure that excessive force is not placed on the Instruments or Probe. Excessive force can result in product failure.
- Prior to the procedure, CT or MRI imaging must be utilized to help define the desired treatment site and define working anatomical landmarks that can be used for mapping access to the treatment site.
- The device should be manipulated only while under fluoroscopic or CT observation.
- DO NOT use this device in the presence of flammable anesthetics, other flammable gases or objects, near flammable fluids such as skin prepping agents and tinctures, or oxidizing agents. Observe appropriate fire precautions at all times. There is a risk of pooling of flammable solutions under the patient or in body depressions and cavities.
- Fluids pooled in the body depressions and cavities of the patient should be mopped up before RFG is used.
- There is a danger of ignition of endogenous gases (e.g., cotton and gauze saturated with oxygen may be ignited by sparks produced during normal use of the RFG).
- Operator may choose to use smoke-plume extraction apparatus, though the indicated procedure is unlikely to produce noticeable smoke
- The Probe is for use WITHOUT a neutral electrode (ie., a grounding pad).
- Discontinue use if inaccurate, erratic or sluggish temperature readings are observed. Use of damaged equipment may cause patient injury.
- Prior to operation, visually inspect the Probe and RF for physical damage, obvious cracks in insulation or loose parts.
- During power delivery, the cable should not come in direct contact with the patient's skin or other patient leads.
- Safety and effectiveness in patients with conditions that are associated with poor bone quality such as osteoporosis have not been established.
CONTRAINDICATIONS
Use of the Intracept Intraosseous Nerve Ablation System is contraindicated in:
- Patients with severe cardiac or pulmonary compromise
- Patients with active implantable pulse generators (e.g. pacemakers, defibrillators)
- Patients where the targeted ablation zone is < 10 mm away from a sensitive structure not intended to be ablated, including the vertebral foramen (spinal canal)
- Patients with active systemic infection or local infection in the area to be treated
- Patients who are pregnant
- Skeletally immature patients (generally ≤ 18 years of age)
WARNINGS
- Do not use if package is opened or damaged as product integrity and/or sterility may be compromised.
- Do not use after the expiration date has passed as product integrity and/or sterility may be compromised.
- Do not re-sterilize or reuse. Re-sterilization or reuse may result in cross contamination, patient infection, or device malfunction.
- Reconditioning, refurbishing, repair or modification of the device to enable further use is prohibited.
- Do not use this product if you have not been properly trained in its use. Physicians using the device should be familiar with the physiology and pathology of the selected anatomy to be treated and be trained in the performance of the chosen surgical technique. Improper surgical use and technique may lead to suboptimal clinical outcomes.
- Read and understand the Instructions For Use ("IFU*) completely prior to use.
- The Intracept System must be used with the Relievant Medsystems RF Generator in order to provide the required treatment parameters.
- Failure of the RFG could result in an unintended increase of output power to the Probe.
- The Intracept RF Probe may interfere and adversely influence the operation of other electronic equipment.
Accordion List
Physicians
Curabitur blandit tempus porttitor. Aenean eu leo quam. Pellentesque ornare sem lacinia quam venenatis vestibulum. Morbi leo risus, porta ac consectetur ac, vestibulum at eros.
Intracept Long Term Outcomes
Curabitur blandit tempus porttitor. Aenean eu leo quam. Pellentesque ornare sem lacinia quam venenatis vestibulum. Morbi leo risus, porta ac consectetur ac, vestibulum at eros.
New Patient Story
Your content goes here. Edit or remove this text inline or in the module Content settings. You can also style every aspect of this content in the module Design settings and even apply custom CSS to this text in the module Advanced settings.
Carousel Doctor
What Physicians Are Saying About Intracept
Carousel Video
Looking for proof?
Hear from the ones living it.
Video Grid
Story
Debbie’s Story
Multiple injections, multiple failed
approaches—nothing worked.
Nullam quis risus eget urna mollis ornare vel eu leo. Cras justo odio, dapibus ac facilisis in, egestas eget quam. Duis mollis, est non commodo luctus, nisi erat porttitor ligula, eget lacinia odio sem nec elit. Lorem ipsum dolor sit amet, consectetur adipiscing elit. Lorem ipsum dolor sit amet, consectetur adipiscing elit. Donec ullamcorper nulla non metus auctor fringilla. Cras justo odio, dapibus ac facilisis in, egestas eget quam.
Duis mollis, est non commodo luctus, nisi erat porttitor ligula, eget lacinia odio sem nec elit. Donec sed odio dui. Curabitur blandit tempus porttitor. Nullam quis risus eget urna mollis ornare vel eu leo. Etiam porta sem malesuada magna mollis euismod. Curabitur blandit tempus porttitor.
Cum sociis natoque penatibus et magnis dis parturient montes, nascetur ridiculus mus. Cras justo odio, dapibus ac facilisis in, egestas eget quam. Vivamus sagittis lacus vel augue laoreet rutrum faucibus dolor auctor. Cum sociis natoque penatibus et magnis dis parturient montes, nascetur ridiculus mus. Sed posuere consectetur est at lobortis.
Next Step forms
Access Instruments CTA
The Intracept® System:
Now with Next-Generation Access Instruments