Getting Back What Pain Has Stolen.
That’s Living Proof.
Meet the Intracept™ Procedure
Chronic low back pain steals so much of life. Now there’s a way to get back to living. The Intracept Procedure: the outpatient procedure for proven relief of chronic vertebrogenic low back pain.
The Only Procedure Designed to Relieve Vertebrogenic Pain
That spontaneous road trip, that special hug, that long-lost workout routine: let’s bring these moments back. The Intracept Procedure is the only procedure specifically designed to target the basivertebral nerve for the relief of chronic vertebrogenic low back pain—letting people lean into life again. That’s living proof.
Vertebrogenic Pain is a Common Source of CLBP
Many patients with CLBP do not know where their pain is coming from: They just know they hurt and haven’t found an answer. Research over the past 30 years has shown that, for many of these patients, their pain is caused by damage to vertebral endplates, the interface between the disc and the vertebral body. This low back pain is vertebrogenic pain—and we’re leading the way to diagnose and treat this condition.
The Intracept™ System:
Now with Next-Generation Access Instruments
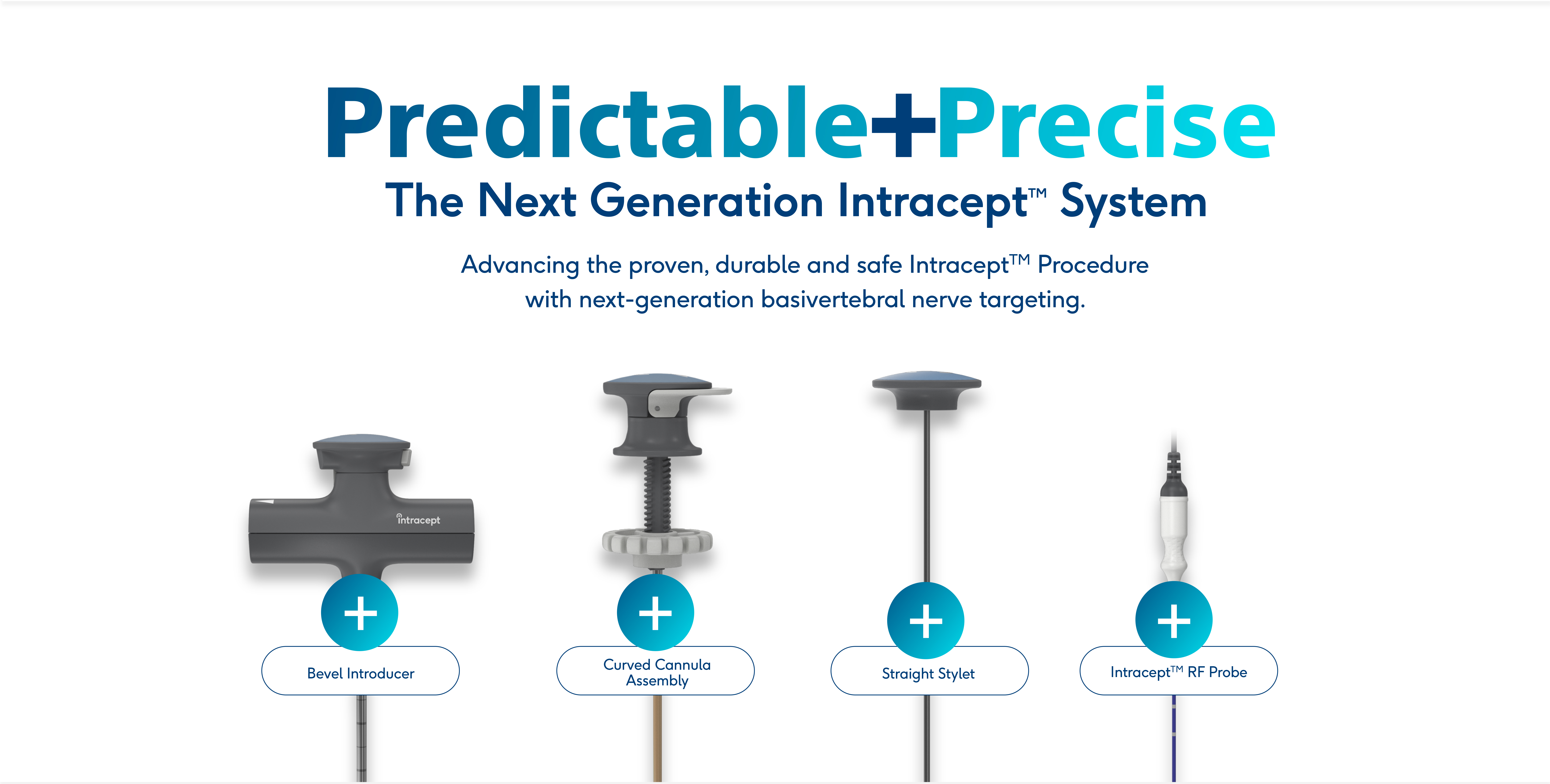
The proven, durable and safe Intracept™ Procedure now features next-generation Intracept Access Instruments for predictable and precise targeting of the basivertebral nerve.
Looking for Proof?
Hear from the Ones Living It.
Real Intracept Procedure patients tell their stories of lasting relief from vertebrogenic pain.
As with any surgical procedure, there are risks and considerations associated with the Intracept Procedure. See important safety information below.
Physicians: See Indications, Contraindications, and Risks
The Intracept Procedure Intraosseous Nerve Ablation System is intended to be used in conjunction with radiofrequency (RF) generators for the ablation of basivertebral nerves of the L3 through S1 vertebrae for the relief of chronic low back pain of at least six months duration that has not responded to at least six months of conservative care, and is also accompanied by features consistent with Type 1 or Type 2 Modic changes on an MRI such as inflammation, edema, vertebral endplate changes, disruption and fissuring of the endplate, vascularized fibrous tissues within the adjacent marrow, hypointensive signals (Type 1 Modic change), and changes to the vertebral body marrow including replacement of normal bone marrow by fat, and hyperintensive signals (Type 2 Modic change).
Use of the Intracept Procedure Intraosseous Nerve Ablation System is contraindicated in:
- Patients with severe cardiac or pulmonary compromise
- Patients where the targeted ablation zone is < 10 mm away from a sensitive structure not intended to be ablated, including the vertebral foramen (spinal canal)
- Patients with active systemic infection or local infection in the area to be treated
- Patients who are pregnant
- Skeletally immature patients (generally < 18 years of age)
- Patients with active implantable pulse generators (e.g., pacemakers, defibrillators)
- Situations where unintended tissue damage may result, based on the clinical assessment by the physician
- Application with electrosurgical instruments NOT tested and specified for use with the Relievant RFG
Patients: See the Indications & Risks Involved
- The Intracept Access Instruments and the Intracept RF Probe are single patient use only. The Introducer Cannula, Diamond Stylet, Bevel Stylet, J-Stylet, Straight Stylet, Drill, and Probe may be used to treat up to a maximum of four (4) vertebrae (L3, L4, L5, S1) on a single patient, while the Curved Cannula may only be used on one (1) vertebra on a single patient.
- As with any surgical instrument, careful attention must be exercised to ensure that excessive force is not placed on the Instruments or Probe. Excessive force can result in product failure.
- Prior to the procedure, CT or MRI imaging must be utilized to help define the desired treatment site and define working anatomical landmarks that can be used for mapping access to the treatment site.
- The device should be manipulated only while under fluoroscopic or CT observation.
- DO NOT use this device in the presence of flammable anesthetics, other flammable gases or objects, near flammable fluids such as skin prepping agents and tinctures, or oxidizing agents. Observe appropriate fire precautions at all times. There is a risk of pooling of flammable solutions under the patient or in body depressions and cavities.
- Fluids pooled in the body depressions and cavities of the patient should be mopped up before RFG is used.
- There is a danger of ignition of endogenous gases (e.g., cotton and gauze saturated with oxygen may be ignited by sparks produced during normal use of the RFG).
- Operator may choose to use smoke-plume extraction apparatus, though the indicated procedure is unlikely to produce noticeable smoke
- The Probe is for use WITHOUT a neutral electrode (ie., a grounding pad).
- Discontinue use if inaccurate, erratic or sluggish temperature readings are observed. Use of damaged equipment may cause patient injury.
- Prior to operation, visually inspect the Probe and RF for physical damage, obvious cracks in insulation or loose parts.
- During power delivery, the cable should not come in direct contact with the patient's skin or other patient leads.
- Safety and effectiveness in patients with conditions that are associated with poor bone quality such as osteoporosis have not been established.
CONTRAINDICATIONS
Use of the Intracept Intraosseous Nerve Ablation System is contraindicated in:
- Patients with severe cardiac or pulmonary compromise
- Patients with active implantable pulse generators (e.g. pacemakers, defibrillators)
- Patients where the targeted ablation zone is < 10 mm away from a sensitive structure not intended to be ablated, including the vertebral foramen (spinal canal)
- Patients with active systemic infection or local infection in the area to be treated
- Patients who are pregnant
- Skeletally immature patients (generally ≤ 18 years of age)
WARNINGS
- Do not use if package is opened or damaged as product integrity and/or sterility may be compromised.
- Do not use after the expiration date has passed as product integrity and/or sterility may be compromised.
- Do not re-sterilize or reuse. Re-sterilization or reuse may result in cross contamination, patient infection, or device malfunction.
- Reconditioning, refurbishing, repair or modification of the device to enable further use is prohibited.
- Do not use this product if you have not been properly trained in its use. Physicians using the device should be familiar with the physiology and pathology of the selected anatomy to be treated and be trained in the performance of the chosen surgical technique. Improper surgical use and technique may lead to suboptimal clinical outcomes.
- Read and understand the Instructions For Use ("IFU*) completely prior to use.
- The Intracept System must be used with the Relievant Medsystems RF Generator in order to provide the required treatment parameters.
- Failure of the RFG could result in an unintended increase of output power to the Probe.
- The Intracept RF Probe may interfere and adversely influence the operation of other electronic equipment.