Level l SMART Trial
Demonstrates Durable Relief1
Purpose
Study Design
- Randomized, double-blind, sham-controlled
- Multi-Center: 15 US and 3 EU sites
- 225 Patients; Randomized to treatment (147) or sham (78) intervention
- Patients were evaluated preoperatively and at 2 weeks and 6 weeks and 3, 6, 12 and 24 months postoperatively
- Skeletally mature patients with chronic (≥ 6 months), isolated lumbar pain who had not responded to at least 6 months of non-operative management
- All patients had Type 1 or Type 2 Modic changes of the treated vertebral bodies
- Outcome Measures: ODI, SF-36, and VAS
- Two pre-defined analysis populations in the study protocol*
Key Findings
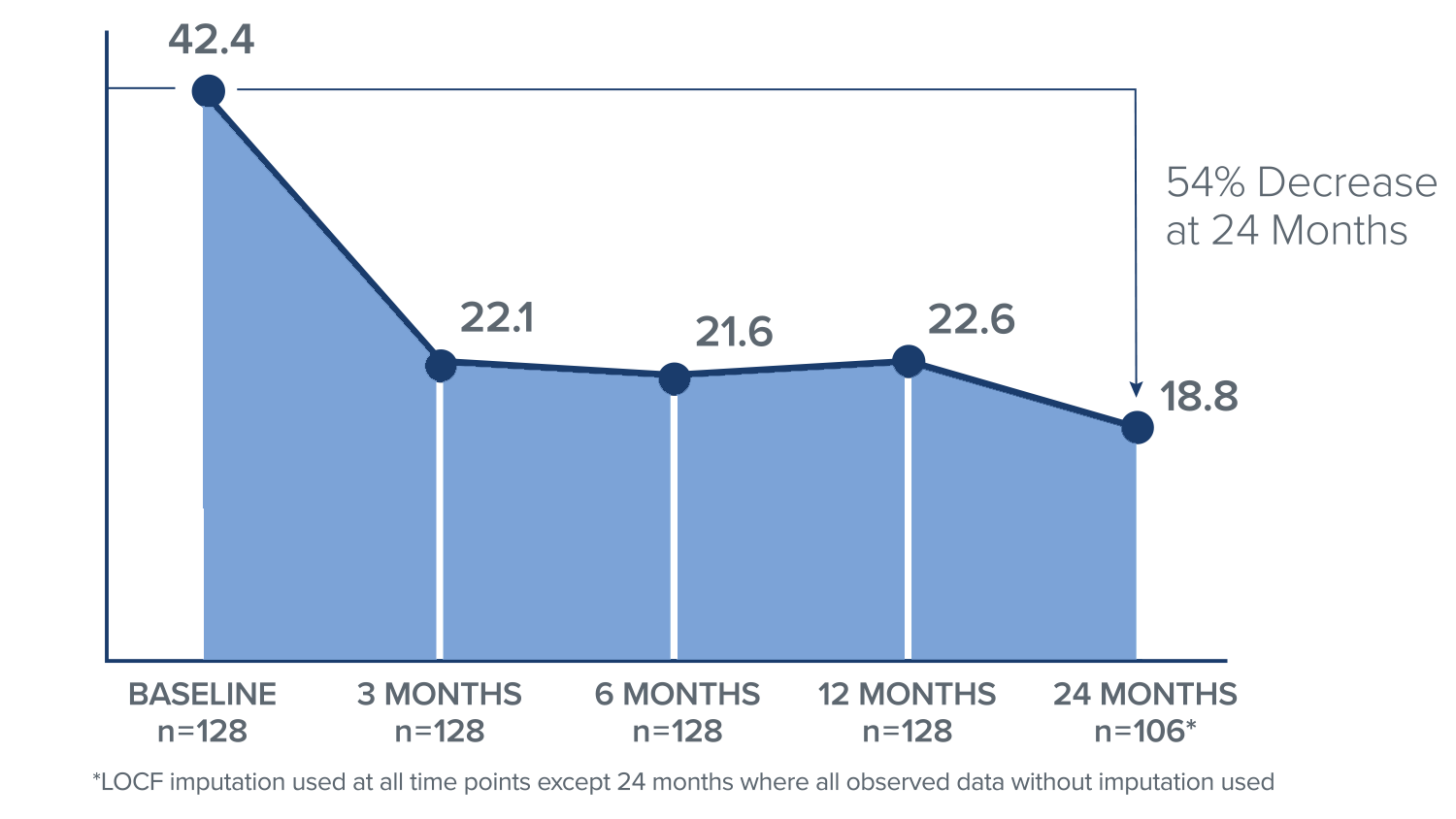
ODI Score
In the Intracept treatment arm, patients reported a 23.6 point mean improvement in ODI, or a 54% decrease from their baseline score at 24 months.
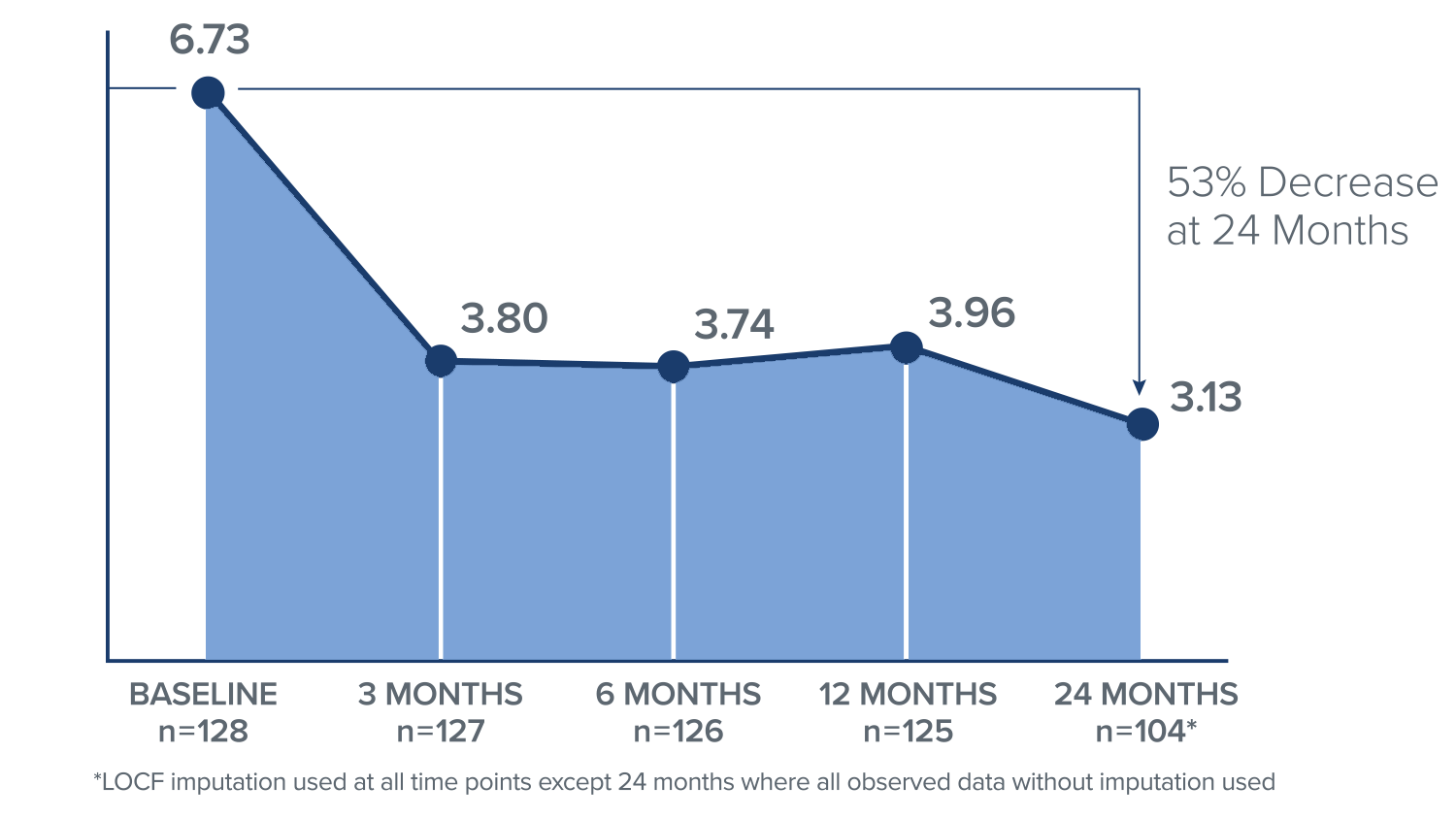
VAS Score
In the Intracept treatment arm, patients reported a 3.6 point mean improvement in VAS, or a 53% decrease from their baseline score at 24 months.
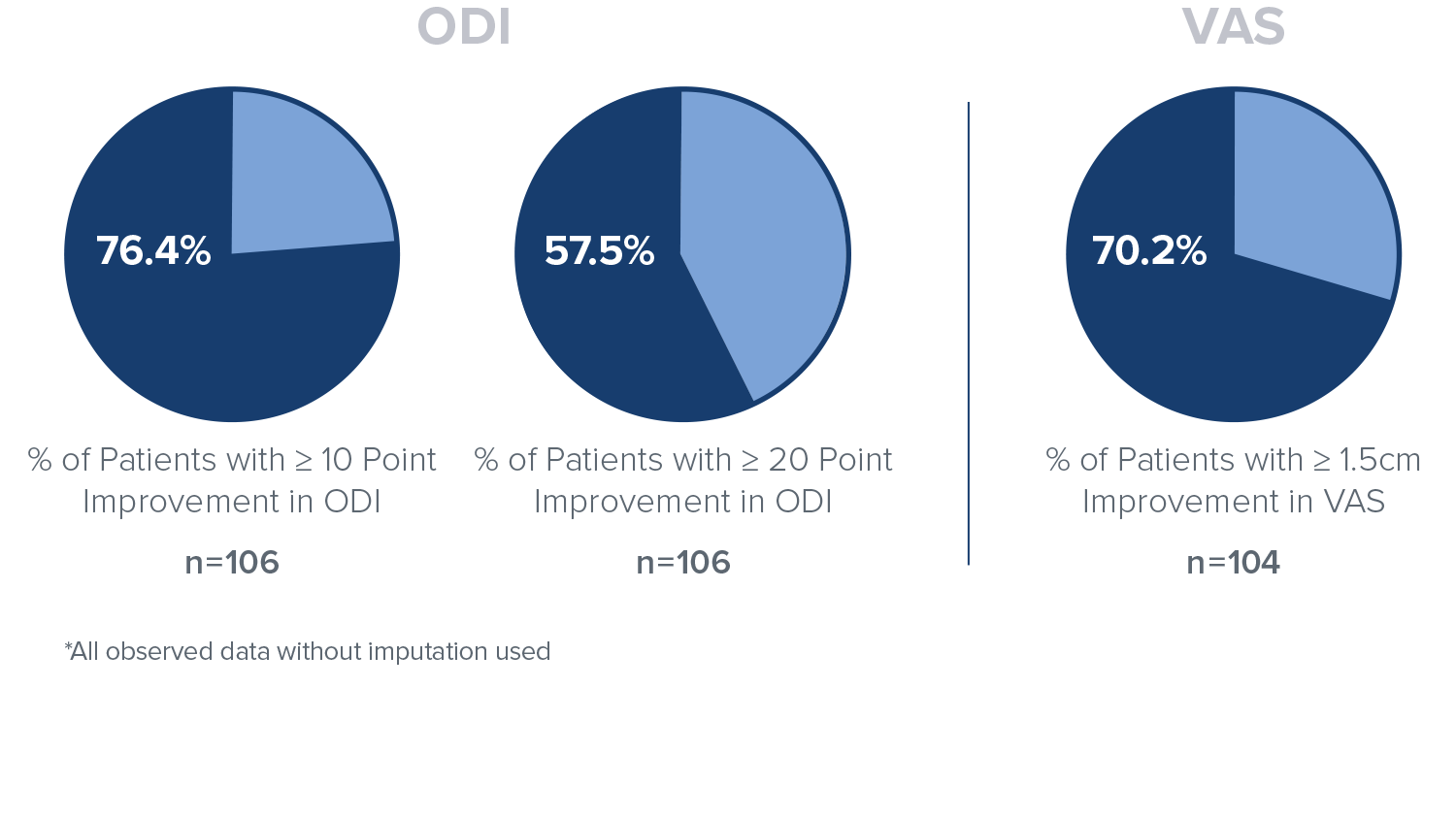
Responder Rate
In the Intracept treatment arm, 76.4% of patients reported a 10 point or greater improvement in ODI, 57.5% of patients reported a 20 point or greater improvement in ODI, and 70.2% of patients reported a 1.5cm or greater improvement in VAS at 24 months.
Safety Results
Conclusion
1. Fischgrund JS, Rhyne A, Franke J, et al. Intraosseous Basivertebral Nerve Ablation for the Treatment of Chronic Low Back Pain: 2-Year Results from a Prospective Randomized Double-Blind Sham-Controlled Multicenter Study. International Journal of Spine Surgery, Vol. 13, No. 2, 2019, pp. 1–10. doi:10.14444/6015
*The intent-to-treat (ITT) population consisted of the patients as randomized and the per-protocol (PP) population excluded any patients in which the procedure could not be carried out, RF generated lesion did not colocate with BVN terminus or who were not compliant with the post-operative protocol.